Difference between revisions of "Dendrochronology"
Tag: Undo |
Tag: Undo |
||
| Line 38: | Line 38: | ||
Growth rings can be recognized because in the beginning of the vegetation period earlywood is being produced with larger, thin-walled cells, whereas at the end of the vegetation period latewood with smaller, thick-walled cells is produced that appears darker. The abrupt change between latewood and earlywood cells is the annual growth boundary that can be usually also recognized without magnification (Fig. 4). | Growth rings can be recognized because in the beginning of the vegetation period earlywood is being produced with larger, thin-walled cells, whereas at the end of the vegetation period latewood with smaller, thick-walled cells is produced that appears darker. The abrupt change between latewood and earlywood cells is the annual growth boundary that can be usually also recognized without magnification (Fig. 4). | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| Line 52: | Line 45: | ||
| − | ''' | + | '''Sampling tree rings''' |
[[File:Figure6 IncrementBorerDENDROCHRONOLOGY.png|thumb|right|Figure 6: Increment borer (8)]] | [[File:Figure6 IncrementBorerDENDROCHRONOLOGY.png|thumb|right|Figure 6: Increment borer (8)]] | ||
| Line 87: | Line 80: | ||
The visual inspection is done on a measuring table with a resolution of 0.01 mm and a microscope. Values are then digitalised, plotted and analysed with computer software (e. g. IML Software T-Tools Pro, Instrumenta Mechanik Labor GmbH). The number of rings and any distinct patterns (frequency of narrow or wide rings) are often what to look out for in this step. | The visual inspection is done on a measuring table with a resolution of 0.01 mm and a microscope. Values are then digitalised, plotted and analysed with computer software (e. g. IML Software T-Tools Pro, Instrumenta Mechanik Labor GmbH). The number of rings and any distinct patterns (frequency of narrow or wide rings) are often what to look out for in this step. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 14:40, 15 November 2024
Quantitative - Qualitative
Deductive - Inductive
Individual - System - Global
Past - Present - Future
In short: Dendrochronology is concerned with the analysis of tree rings, whose chronological sequence can be used to date wood samples and infer information on environmental conditions, stressors and their effects on the plant species.
Contents
Visualisation of a typical result
Background
Various scientists had been aware of tree rings and their connection to climate for several centuries already, however the term ‘dendrochronology’ was coined by A. E. Douglass from the University of Arizona in 1929. He used the method of tree-ring dating to determine the age of ancient indigenous buildings, which helped the Navajo peoples’ compensatory claims to succeed (2). Tree ring analysis or dendrochronology is applied in many disciplines like climatology, archaeology, biology, hydrology and forestry. It can be applied to any plant species that has a woody growth type (including shrubs) and any woody parts of the plants (including branches, twigs and roots). Seasonal growth, the number of growth rings and the ring widths, is determined by the interaction of genetic and environmental factors. For example, a poplar forms wider rings than a bilberry growing under the same climatic conditions (3). Furthermore, in order to analyse seasonal growth rings only one environmental factor must dominate in limiting the growth. This limiting factor can be precipitation in arid, semi-arid or tropical parts of the world or temperature like in the temperate regions (e. g. dry and wet season in tropical forests, winter and summer in temperate forests).
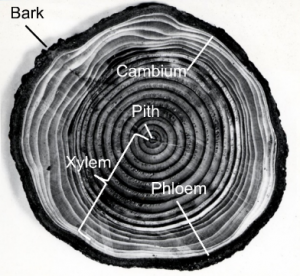
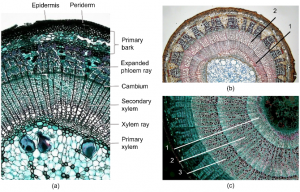
The structure we see as rings is a sequence of earlywood and latewood in the secondary xylem tissue of a stem. Secondary growth reflects an increase in thickness, or lateral additions of new tissue, and essentially this secondary xylem is an important resource known as wood. The xylem is the water and nutrient conducting tissue in vascular plants. Cells for the secondary xylem are generated by the cambium layer. The cambium is a zone of undifferentiated cells that produce cells of the xylem to the centre of the stem and cells of phloem to the outer part of the stem. The phloem is the plant tissue for transport of sugars (assimilates). Xylem and phloem together form the vascular system of plants throughout the stems, branches and roots. For every vegetation period, governed by one limiting environmental factor, the cambium generates xylem to the inside of the stem and phloem to the outside. The cambium will generate more secondary xylem than phloem and old outer phloem tissue will be crushed and eventually become bark. This is why woody species accumulate more and more secondary xylem each year and not secondary phloem. The oldest rings are close to the centre of the tree stem, the youngest near the cambium and bark (Fig. 3).
Growth rings can be recognized because in the beginning of the vegetation period earlywood is being produced with larger, thin-walled cells, whereas at the end of the vegetation period latewood with smaller, thick-walled cells is produced that appears darker. The abrupt change between latewood and earlywood cells is the annual growth boundary that can be usually also recognized without magnification (Fig. 4).
What the method does
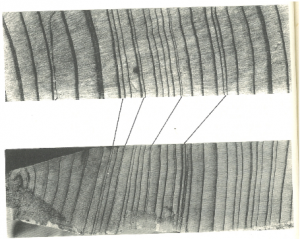
Dendrochronologists are interested in the chronological sequence of growth rings. In order to establish a dendrochronology of a certain area, scientists compare tree rings of individuals of one species. For this purpose, this species must only add one ring per growing season, the growth-limiting climatic factor must vary in intensity from year to year, and should be uniformly effective over a large geographic area (i. e. the relative annual difference in intensity is similar throughout the macroclimatic region). The variation in the growth-limiting factor must be reflected in the width of the tree rings. If these conditions are fulfilled scientists can recognize specific sequences that are of use for cross-dating or matching ring patterns between specimens (see (2) for an introduction and Fig. 5). Which species makes most sense to study depends on many factors, like the research objective, conditions in the study area, abundance and properties of the species.
Sampling tree rings
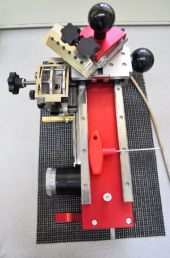
Dendrochronology is a method of data gathering and analysis. The findings can then be interpreted, for instance in regards to tree wellbeing or climate. To collect the data, core sampling of living trees is performed with an increment borer (Fig 6). The increment borer consists of a handle, a borer and an extraction spoon. This tool removes a sample from the stem leaving a hole of about 5 mm wide half the stem diameter deep. This hole quickly fills with sap; however, it is still an invasive method that could allow parasites or fungi to enter the bore hole. Therefore, the tool should be cleaned after each usage and sampling should be done with care and only when it is necessary.
Selection of sampling sites and individual trees depends on the research question. Usually, researchers are interested in investigating variability within years. To this end, trees that do not have underground water access are sampled. Trees growing under these conditions will have so called sensitive ring sequences useful for dating, in contrast to trees with underground water access, that will have so called complacent ring sequences with equally narrow or wide rings. Core samples are therefore preferably taken from trees growing on the top of the slope. The increment borer is placed at a 90° angle on the stem facing the side slope. This means neither facing uphill nor downhill to avoid areas where the ring patterns are likely to be distorted
Sampling
It is important to take field notes on the immediate environment of the sampled tree, since this information on growth-affecting parameters will be necessary for interpretation later (e. g. Table 1). When possible, samples are taken well below the first branch facing the side of the slope. After choosing a suitable area on the stem, put beeswax on the borer tip and place the tip of the increment borer at a 90° angle on the stem. Turn the handle clockwise to drill into the tree. Aim the borer at the pith. After the borer has reached the desired depth (middle of the stem), insert the extraction spoon into the borer from the handle end. When the extraction spoon is fully inserted between the wood core and the metal sides of the borer, give the borer a full turn counter clockwise. Remove the extraction spoon with the sample from the borer. Place the sample in the envelope or mount (most recent rings orientated to the right side of the mount). Remove the borer from the tree by turning it counter clockwise. Clean the inside with WD 40.
Table 1: Example of site data sheet (DBH = diameter breast height at 130 cm)
| Date | Observer | Sample ID | Location | Inclination |
| Vegetation | Species sampled | DBH (in cm) | Distance to surrounding trees | Canopy cover |
| soil type | Remarks (disturbance, pests, distance to open water, distance to path…) |
Preparation
The core sample will shrink and bend while drying. Therefore, place the core sample in a slotted mount and fixate with pushpins for air drying. After drying for about one week, cut off the core top with a core microtome to create a smooth straight surface (Fig. 7).
Analysis
The visual inspection is done on a measuring table with a resolution of 0.01 mm and a microscope. Values are then digitalised, plotted and analysed with computer software (e. g. IML Software T-Tools Pro, Instrumenta Mechanik Labor GmbH). The number of rings and any distinct patterns (frequency of narrow or wide rings) are often what to look out for in this step.
Strengths and Challenges
An advantage of this method is, that it can be applied to all woody (parts of) plants. One can sample the living organisms by taking only a small-diameter core sample. After dendrochronologically analysing a representative amount of the population, one can statistically calculate an estimate, which allows the researcher to assume the age of other branches/roots/etc. from that population based on their diameter without sampling them. The method can also be applied to tree stumps of logged trees or wood used as building material and charcoal samples to date an archaeological site.
Picking a representative sample of plants to account for the natural variability as well as taking the necessary number of samples can be difficult, for example in steep terrain. The high pressure from competition in dense plant coverage and also injuries and diseases can affect a plants growth and obscure the influence of the climatic factor. Decayed wood is not useful for dendrochronological analysis. One should also consider the hardness of the wood of the species of interest. Bore holes are more difficult to drill into certain species, like Hornbeam and Robinia.
Distinguishing the growth rings is another challenge of varying difficulty depending on the species. Coniferous trees tend to have very clear growth rings. With oak and beech, the rings usually also are fairly clear, whereas the growth rings of hornbeam are way less easy to see.
Furthermore, abnormalities can occur in the ring patterns, which one has to be aware of when cross dating several samples. The ring of a year with extremely little growth could be locally absent at the sampled point of the stem. It is not missing on the whole stem, widths of rings vary lightly across the stem of a tree. Similarly, double or false rings can form in parts of the stem, meaning that there are two dark bands from one year. Causes for this are not well understood yet. Taking two samples of the same tree decreases the chance that any such irregularity is missed in analysis.
Normativity
Although the bore hole is relatively small in diameter, this method still is invasive and pathogens could enter the plant system through the wound caused by the borer, potentially leading to disease or even death of the plant due to the research. As a guiding principle for ecological sampling, researchers should cause as little disturbance as possible. One should therefore consider alternatives and choose the least invasive method applicable and accessible.
Uncertainty results from the subjective experience and view of the researcher. The quality and clarity of the core samples depends on the experience of the researcher with taking and preparing samples. At the analysis step, different people might count the growth rings differently. Especially if multiple researchers are working on the same project, it thus is necessary to agree on and document how is counted, in order to ensure comparability and reproducibility.
One practical application of dendrochronology was the Navajo Land Claim Project. For 18 years from 1951 onwards, the University of Arizona’s Laboratory of Tree-Ring Research and the Navajo Tribe worked together, closely linking science and society. They took thousands of samples from living trees and old Navajo hogans in and around the Navajo Reservation. Cross-dating the samples provided evidence, that the Navajo peoples had formerly occupied areas outside their reservation, which they had been evicted from (10). Therefore, the United States Land Claims Commission acknowledged the tribe to be eligible for compensation (2).
Outlook
Tree ring analysis is also applied to determine the health status of individuals or forests and effects of climate change (dendroclimatology). Based on the growth of these long-lived species one can interfere with past environmental conditions and current stressors. Due to climate change, years with droughts increase but on the other hand the length of growing seasons also increases. How this affects different species and forest composition can be investigated through tree ring analysis. Thus, dendrochronology can help foresters in making their woods future-proof and sustainable. It could also play a role in the selection and management of city trees. On a local or regional scale, insights gained from tree ring analysis might inform climate adaptation measures which involve plants.
New, less invasive tools are being developed for collecting data on growth rings of living trees. In forestry, even thinner borers are in use already, reducing the possible negative impacts on plant health.
Key publications
Härdtle, W., Niemeyer, T., Assmann, T., Aulinger, A., Fichtner, A., Lang, A., ... & von Oheimb, G. (2013). Climatic responses of tree-ring width and δ13C signatures of sessile oak (Quercus petraea Liebl.) on soils with contrasting water supply. Plant ecology, 214 (9), 1147-1156.
Heklau, H., & von Wehrden, H. (2011). Wood anatomy reflects the distribution of Krascheninnikovia ceratoides (Chenopodiaceae). Flora-Morphology, Distribution, Functional Ecology of Plants, 206 (4), 300-309.
Mausolf, K., Härdtle, W., Jansen, K., Delory, B. M., Hertel, D., Leuschner, C., ... & Fichtner, A. (2018). Legacy effects of land-use modulate tree growth responses to climate extremes. Oecologia, 187 (3), 825-837.
Schweingruber, F. H. (2001). Dendroökologische Holzanatomie. Eidgenössische Forschungsanstalt WSL, Haupt, Bern, Stuttgart, Wien.
Furthermore, see references.
References
(1) Foster, K. (2013a). Dendrochronology Lab at ICRAF Nairobi. Online at: https://www.flickr.com/photos/icraf/9245105189/in/photostream/ (08.02.2023).
(2) Stokes, M. A. & Smiley, T.L. (1996). An introduction to tree-ring dating. University of Arizona Press.
(3) Schweingruber, F. H. (1988). Tree rings: basics and applications of dendrochronology. Kluwer, Dordrecht, Boston, Lancaster, Tokyo.
(4) USDA (1968). Growth rings. Cross section of tree. USDA Forest Service, Pacific Northwest Region, State and Private Forestry, Forest Health Protection. Portland Station Collection, La Grande, Oregon. Online at: https://www.flickr.com/photos/151887236@N05/27086026918 (08.02.2023).
(5) Fayette, A., Reynolds, M. S. (2014a). Woody Dicot Stem: One Year Tilia. Berkshire Community College Bioscience Image Library. Online at: https://www.flickr.com/photos/146824358@N03/34315403424 (08.02.2023).
(6) Fayette, A., Reynolds, M. S. (2014b). Woody Dicot Stem: Three Year Old Tilia. Berkshire Community College Bioscience Image Library. Online at: https://www.flickr.com/photos/146824358@N03/34714986052 (08.02.2023).
(7) Fayette, A., Reynolds, M. S. (2014c). Woody Dicot Stem: Two Annual Rings in Tilia. Berkshire Community College Bioscience Image Library. Online at: https://www.flickr.com/photos/146824358@N03/35031940521 (08.02.2023).
(8) Beentree (2006). Pressler drill work on Populus alba. Online at: https://commons.wikimedia.org/wiki/File:Pressler_drill_5_beentree.jpg (08.02.2023).
(9) Foster, K. (2013b). Dendrochronology Lab at ICRAF Nairobi. Online at: https://www.flickr.com/photos/icraf/9247888378/in/photostream/ (08.02.2023).
(10) Stokes, M.A., Smiley, T.L. (1963). Tree-ring dates from the Navajo Land Claim I. The northern sector. Tree-Ring Bulletin, 25(3-4), 8-18.
The authors of this entry are Heike Zimmermann and Fabienne Friedrichs.