Difference between revisions of "Mammals"
(Created page with "300px|thumb|right|[[Design Criteria of Methods|Method Categorisation:<br> '''Quantitative''' - Qualitative<br> '''Deductive''' - '''Induc...") |
|||
| (2 intermediate revisions by the same user not shown) | |||
| Line 12: | Line 12: | ||
| || '''Line transects''' || '''Trapping''' || '''Counting dung''' | | || '''Line transects''' || '''Trapping''' || '''Counting dung''' | ||
|- | |- | ||
| − | |'''Carnivores''' || | + | |'''Carnivores''' || || || + |
|- | |- | ||
|'''Sea mammals''' || + || || | |'''Sea mammals''' || + || || | ||
| Line 18: | Line 18: | ||
|'''Primates''' || + || || | |'''Primates''' || + || || | ||
|- | |- | ||
| − | |'''Ungulates''' || * || | + | |'''Ungulates''' || * || || * |
|- | |- | ||
| − | |'''Bats''' || | + | |'''Bats''' || || || |
|- | |- | ||
| − | |'''Rodents''' || | + | |'''Rodents''' || || *|| + |
|- | |- | ||
|'''Rabbits, hares & pikas''' || + || + || * | |'''Rabbits, hares & pikas''' || + || + || * | ||
| Line 28: | Line 28: | ||
|'''Insectivores & elephant shrews''' || ||* || | |'''Insectivores & elephant shrews''' || ||* || | ||
|- | |- | ||
| − | |'''Edentates''' || +|| || | + | |'''Edentates''' || +|| || |
|- | |- | ||
|} | |} | ||
| Line 79: | Line 79: | ||
For '''Strip and line transects''', the largest bias lies in the number of animals that could not be counted as they were overlooked or ran away before they were counted. This number is hard to quantify and might be underestimated even by using the above-mentioned test (Sutherland 1996). | For '''Strip and line transects''', the largest bias lies in the number of animals that could not be counted as they were overlooked or ran away before they were counted. This number is hard to quantify and might be underestimated even by using the above-mentioned test (Sutherland 1996). | ||
| − | '''Trapping''' of small mammals might lead to an overestimation of individual number as the activity of small mammals strongly increases on nights when it is warm, dry and dark (Lockhart & Owings | + | '''Trapping''' of small mammals might lead to an overestimation of individual number as the activity of small mammals strongly increases on nights when it is warm, dry and dark (Lockhart & Owings 1974, Mystkowska & Sidorowicz 1961, Vickery & Bider 1978). This method also poses the largest ethical challenges as mammals should not be kept in a cage for too long. Otherwise there is a threat of the animal dying from stress, from too hot/ too cold temperatures or starving to death. |
'''Camera trapping''' is mostly applicable for mammals of a similar heat signature as a deer as most camera traps with infrared sensors are produced for the hunting market. This makes them suitable for most large to midsized mammals. However, for average cameras, animals should not weigh less than 1kg (100 g for high-end cameras). The reliability of camera traps declines when the mammal is further away than 2 m (WWF 2023). | '''Camera trapping''' is mostly applicable for mammals of a similar heat signature as a deer as most camera traps with infrared sensors are produced for the hunting market. This makes them suitable for most large to midsized mammals. However, for average cameras, animals should not weigh less than 1kg (100 g for high-end cameras). The reliability of camera traps declines when the mammal is further away than 2 m (WWF 2023). | ||
Latest revision as of 10:06, 16 December 2023
Quantitative - Qualitative
Deductive - Inductive
Individual - System - Global
Past - Present - Future
In short: Line-transects, trapping and counting dung are selected methods for counting mammals.They are suitable for many different species of mammals. Additional methods for counting mammals can be found in Sutherland (1996).
Table 1. The use of the methods strip and line transects, trapping and counting dung for different groups of mammals (modified after Sutherland 1996). *method usually applicable +method often applicable.
| Line transects | Trapping | Counting dung | |
| Carnivores | + | ||
| Sea mammals | + | ||
| Primates | + | ||
| Ungulates | * | * | |
| Bats | |||
| Rodents | * | + | |
| Rabbits, hares & pikas | + | + | * |
| Insectivores & elephant shrews | * | ||
| Edentates | + |
Contents
Strip and line transects
What the method does
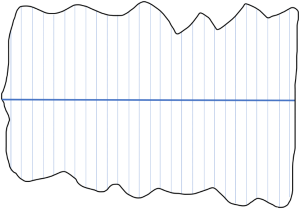
Transects are a suitable method for counting conspicuous species. Strip transects consist of a baseline that is drawn on a map and crosses the whole territory that should be mapped. Transect lines cross the baseline in a perpendicular angle in a way that the whole territory is covered with transects. Each transect has the same width (Sutherland 1996). Ecological Census Techniques - a Handbook. P. 178-180. Cambridge University Press. 172-176.. For instance, if the baseline is 20 km long and has 50 transects, each transect is 400 m wide (Figure 1).
The transects are numbered and the transects that are surveyed are randomly selected. For navigation, the transects are drawn onto a map (Sutherland 1996). To get an index of relative abundance, a constant speed needs to be kept while driving or walking and individuals should only be counted within a certain distance. Usually, a distance between 200 m and 400 m to each side is selected. For some species, using a boat or vehicle is recommended as they might be scared away by a human walking before it is possible to count them as walking is too slow and conspicuous. If a vehicle is used, it should be one that raises the observer so that it is easier to spot animals in thick vegetation (Sutherland 1996). Training the observers is of high importance to not miss out on too many individuals. To get an estimate of how many individuals are missed, two teams of observers can walk the same transect at the same time and compare their results at the end (Sutherland 1996). For nocturnal species, it is recommended to look at the reflection of light from the back of the retina (“spotlighting”). Therefore, a torch or a headlight is used. Spotlighting is often done from a vehicle (Sutherland 1996).
Strengths and challenges
For small areas of less than 1000 km2, this method is the most suitable, especially for open habitats such as savannah or tundra. A major challenge is to not scare animals away before they are counted (Sutherland 1996).
Trapping
What the method does
Trapping is a useful method for the census of small terrestrial mammals. There are four kinds of traps with different advantages and disadvantages. Sherman traps are in collapsible form and can be bought in many different sizes. Bromilow traps are suitable for small and medium macropods. Longworth traps are mainly used in Britain. They are effective but bulky. Pitfall traps can only be used where a hole can be dug in the ground. They should have drain holes and be sheltered from the sun to prevent overheating (Sutherland 1996). Traps are placed in pairs with the edge of the trap flush with the ground. Moreover, they should be marked, so that they can be found again. Placing traps along logs or runs in the vegetation is most successful. For arboreal species such as squirrels, traps should be attached to thick branches. In the case of aquatic species, traps can be fixed onto floating platforms. Here, it is important that the platform still floats after a mammal is caught (Sutherland 1996). If more than 60% of traps are occupied, more traps should be put into place. However, the maximum number of traps that can be checked in a day lies between 200-300. Usually, traps are checked twice per day, at dusk and at dawn. If shrews are expected to get caught, traps should be checked every 4 hours as they would die otherwise. This applies even if shrews are not the target. Additionally, they need to be provided with a lot of live food as they have to eat their own weight within 24 hours to survive. Therefore, fly castors from fishing shops are recommended (Sutherland 1996). Traps should contain bait to lure individuals of the target species. As bait, seeds are a good option for many species as they are dry and do not decay fast. For rodents, peanut butter mixed with oats and water is a good bait. For piscivorous species, fresh or tinned fish without sauce is used (Sutherland 1996). To minimize edge effects, traps are placed in a square grid with a spacing of 5 m to 20 m. Each trap should be marked with a cane for better visibility. To estimate population sizes, the mark-release-recapture method is recommended (Montgomery 1987). Therefore, the individuals are marked by clipping the guard hair so that the hair underneath is revealed. If the same individual is caught again the following time, it is not counted. To get a comparable population estimate across sites, the number of caught individuals is divided by the number of trapping nights. Therefore, the trap density needs to be the same in both sites (Erlinge 1983).
Strengths and challenges
This method is very time consuming, especially if there is only a low density of individuals. However, a lot of data can be obtained from each individual such as weight, sex rations and reproductive state. Country specific laws need to be followed when working with mammals. Moreover, there is the risk of contracting diseases such as rabies. Holding animals for a longer time during the breeding period might result in abortion or desertion of the young (Sutherland 1996).
Camera trapping
What the method does
Modern camera traps usually consist of a digital compact camera sensor wired up to a passive infrared sensor that registers infrared radiation from warm-blooded animals. A soon as such an animal is registered by the sensor, the camera takes a picture. However, there are also other methods for the camera to get triggered by an animal such as trip-wires, pull-wires, pressure plates, lasers or microwave sensors (WWF 2023). Camera traps are getting better and are gaining additional features. In most cases, traps are placed in the wild and the SD-card is checked when it is full. However, with networked camera traps it is even possible to observe animals almost in real time if the trap is connected to the phone or satellite network (WWF 2023). The advantage of camera traps is that they do not disturb animals and can produce a lot of data without much work. The most labor-intensive part is checking all the photographs for relevant species. However, analysis of thousands of images from camera traps is also getting easier with new software tools and statistical models (WWF 2023). ty needs to be the same in both sites (Erlinge 1983).
Strengths and challenges
The biggest advantage of camera traps is that they are much less labor-intensive than the other methods in this article. Moreover, they do not cause harm to the animals as they are not caught in a “real” trap where they would have to wait until they are released again (WWF 2023). Challenges can occur in the form of technical issues. For cameras that are regularly checked and save the photos on an SD-card, issues occur when the SD card is full and not changed in time. The biggest challenge for networked cameras is that they could lose the connection to the network. An overall challenge for all camera traps is that they might get stolen by passers-by as they are quite valuable. This issue is directly connected to the fact that the costs for a camera that is suited to scientific purposes range from around 300 Euro to 1000 Euro (WWF 2023). As usually several cameras are needed for observations in an area, this can sum up to a high amount of money.
Counting dung
What the method does
Counting dung is a good method to identify a certain mammal species as dung is far more conspicuous than the animal itself (Wood 1988). For counting dung, following a line transect is recommended (see Chapter 1). Dung can usually be found in aggregations. The time span during which dung persists can strongly differ depending on the weather, especially rain, dung composition, amount of fiber and the number of dung beetles present (Sutherland 1996). This problem can be solved by only counting the dung from a certain time interval. Therefore, dung is completely removed from marked areas. During the next visit, only the new dung is counted in these areas (Sutherland 1996).
Strengths and challenges
For many mammals that are rather evasive, counting dung is the only applicable method to census them. However, for closely related species, this method is not applicable to distinguish them as their dung might be too similar (Sutherland 1996).
Normativity
For Strip and line transects, the largest bias lies in the number of animals that could not be counted as they were overlooked or ran away before they were counted. This number is hard to quantify and might be underestimated even by using the above-mentioned test (Sutherland 1996).
Trapping of small mammals might lead to an overestimation of individual number as the activity of small mammals strongly increases on nights when it is warm, dry and dark (Lockhart & Owings 1974, Mystkowska & Sidorowicz 1961, Vickery & Bider 1978). This method also poses the largest ethical challenges as mammals should not be kept in a cage for too long. Otherwise there is a threat of the animal dying from stress, from too hot/ too cold temperatures or starving to death.
Camera trapping is mostly applicable for mammals of a similar heat signature as a deer as most camera traps with infrared sensors are produced for the hunting market. This makes them suitable for most large to midsized mammals. However, for average cameras, animals should not weigh less than 1kg (100 g for high-end cameras). The reliability of camera traps declines when the mammal is further away than 2 m (WWF 2023).
Counting dung is a suitable method for giving an estimate of animal densities without disturbing them too much. However, in some cases where dung of different species looks very similar, it might be attributed to the wrong species (Sutherland 1996).
Outlook
In the future, census of mammals will most likely rely more and more on DNA sampling. The problem of dung looking too similar between closely related species is becoming less important as it is possible to distinguish dung based on mitochondrial DNA (Joshi et al. 2022). For a study in the Indian Himalayan Region, DNA was extracted from 3363 dung samples collected in six regions in India and amplified via PCR by using seven different mitochondrial primer pairs. DNA was sequenced and sequences searched using the NCBI BLAST search for species determination. Of those samples, 65% yielded good quality samples and 41 species of mammals were determined (Joshi et al. 2022). Telemetry is another method that was further developed in recent years, although it has been used already for decades in wildlife ecology. Telemetry is “the system of determining information about an animal through the use of radio signals from or to a device carried by the animal” (Gutema 2015). Recently, cost-effective and automated wildlife tracking systems were developed matching to the relevant ecological contexts in which mammals live. This makes it easy to generate a large amount of data with little resources (Nathan et al. 2022). However, especially small mammals such as rabbits, voles and lemmings can be impacted in their behavior through tags. Small mammals with tags are likely to be impaired in their movement and show a decreased digging ability (Gutema 2015). In the future, this problem might be of less importance if smaller and lighter tags are developed.
Key publications
Theoretical:
Sutherland, W. J., Editor (1996). Ecological Census Techniques - a Handbook. Cambridge University Press. 260-280.
Empirical:
Joshi, B. D., Singh, S. K., Singh, V. K., Jabin, G., Ghosh, A., Dalui, S., ... & Thakur, M. (2022). From poops to planning: A broad non-invasive genetic survey of large mammals from the Indian Himalayan Region. Science of The Total Environment, 853, 158679.
References
(1) Erlinge, S. (1983). Demography and dynamics of a stoat Mustela erminea population in a diverse community of vertebrates. The Journal of Animal Ecology, 705-726.
(2) Gutema, T. M. (2015). Wildlife radio telemetry: use, effect and ethical consideration with emphasis on birds and mammals. Int J Sci Basic Appl Res, 24(2), 306-313.
(3) Joshi, B. D., Singh, S. K., Singh, V. K., Jabin, G., Ghosh, A., Dalui, S., ... & Thakur, M. (2022). From poops to planning: A broad non-invasive genetic survey of large mammals from the Indian Himalayan Region. Science of The Total Environment, 853, 158679.
(4) Lockart, R.B. & Owings, D.H. (1974). Seasonal changes in the activity pattern of Dipodomys spactabilis. Journal of Mammology. 55, 291-297.
(5) Montgomery, W. I. (1987). The application of capture-mark-recapture methods to the enumeration of small mammal populations. In Symposia of the Zoological Society of London (Vol. 58, pp. 25-57).
(6) Mystkowska, E. & J. Sidorowicz. 1961. Influence of the weather on captures of Micro Mammalia. II. Insectivora. Acta Theriol., 5:263-273.
(7) Nathan, R., Monk, C. T., Arlinghaus, R., Adam, T., Alós, J., Assaf, M., ... & Jarić, I. (2022). Big-data approaches lead to an increased understanding of the ecology of animal movement. Science, 375(6582), eabg1780.
(8) Sutherland, W. J., Editor (1996). Ecological Census Techniques - a Handbook. P. 178-180. Cambridge University Press. 172-176.
(9) Vickery, W. L., & Bider, J. R. (1978). The effect of weather on Sorex cinereus activity. Canadian Journal of Zoology, 56(2), 291-297.
(10) Wood, D. H. (1988). Estimating rabbit density by counting dung pellets. Wildlife Research, 15(6), 665-671.
(11) WWF (2023). Camera trapping for conservation. https://www.wwf.org.uk/project/conservationtechnology/camera-trap (last access on 08/06/2023)
The author of this entry is Anna-Lena Rau.