Amphibians
Quantitative - Qualitative
Deductive - Inductive
Individual - System - Global
Past - Present - Future
In short: Drift fencing, netting and transect/ patch sampling are methods for collecting data on the abundance and species richness of amphibians.
Table 1. Suitability of the methods drift fencing, trapping and transect/ patch sampling for different groups of species (according to Sutherland 1996). *method usually applicable +method often applicable.
| Drift fencing | Trapping | Transect and patch sampling | |
| Species that migrate to breed at specific localities | * | * | |
| Species that are abundant in a restricted area | + | ||
| Species that are dispersed over a wide area | * |
Contents
Drift fencing
What the method does
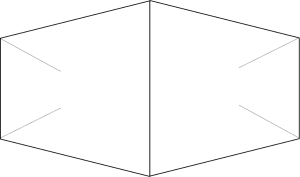
For drift fencing, a fence of 1 m height is built out of plastic or metal in a perpendicular direction to an amphibian migration route such as a water course. It should be placed at a distance of 2 to 5 meters from the water’s edge and buried underground to a depth of about 20 cm, so that no animals can crawl underneath it (Figure 1).

Inside and outside the fence, pitfall traps are dug into the ground along the fence with a facing of 3 m to 5 m. These traps can be plastic buckets or metal cans. They should have small holes at the bottom to prevent caught animals from drowning if water gets into the trap. Moreover, the pitfall traps should also contain cover objects such as broken flower pots under which the animals can seek shelter. Pitfall traps should be checked daily, as they attract predators of amphibians. Moreover, amphibians could dry out and die in hot weather. The best times to check the traps are 2 to 3 hours after dusk and just after daybreak.
Strengths and challenges
Drift fencing is a useful technique for capturing a large number of amphibians in a specific area, and it allows researchers to obtain data on the species composition, population size, and migration patterns of amphibians in the area. Drift fencing has the advantage that large numbers of animals can be captured in a relatively short period of time. As the whole population can be sampled using a drift fence, the size of the population can be determined using this method. Moreover, this method is useful for determining the migration direction, sex, age and body-size, differences in migration times and time spent at the breeding site. The challenges of this method are that drift fences are expensive to build and labor-intense. Additionally, they can easily be destroyed by animals or children and are not suitable for all kinds of habitats. Overall, drift fencing can be an effective technique for capturing large numbers of amphibians. However, it should be used in combination with trapping or collecting of animals in the breeding site itself as there are some species that stay so close to the breeding site that they might not be caught in the pitfall traps.
Trapping
What the method does
Trapping is a method for sampling amphibians that is suitable for water bodies and soil areas.
Aquatic amphibians In water bodies, funnel traps made out of plastic water bottles can be used for sampling amphibians and their larvae. The trap should either offer an access to the water surface or be only partially submerged, because many species need air from the water surface to breathe. Larger funnel traps are made of net material. Different funnel-trap designs have varying efficacies, as shown in studies by Buech and Egeland (2002a).
Terrestrial amphibians Terrestrial amphibians can be sampled with pitfall traps such as those used for drift fencing (see previous chapter). Another option is to set out cover objects such as pieces of wood underneath which the amphibians such as salamanders can hide. These cover objects should be positioned to intercept the animals when they are walking their usual routes. For instance, salamanders are moving from deeper water during the day to shallow ponds at night.
Strengths and challenges
Trapping is a cheap and simple way to catch amphibians, but it can also take a lot of effort. Unless there is a high density of individuals, it might be hard to catch many. Traps can also hurt amphibians or make them suffer from the heat inside, so it's important to check the traps often and release the animals quickly, especially on sunny and warm days. However, just using traps is not sufficient to measure population size accurately. This is only possible by conducting a mark-and-recapture-study. This means catching some animals, marking them, and releasing them, and then catching some more later on. By seeing how many of the second group are marked, it is possible to figure out how many animals the population comprises.
Transect and patch sampling
What the method does
Transect and patch sampling are ways to find amphibians there are and where they are living in an area. Transect sampling involves walking along a line, like a trail or a river, and counting all visible amphibians. This can give an estimate of the location of amphibians in an area. Patch sampling involves looking in a specific area, like a pond or a wetland, and counting the amphibians that can be found there. Therefore, one or more marked out patches are laid out across a habitat. They usually have a square format and are ideally randomly distributed. If there is a big number of evenly distributed squares, this enables statistical inferences about the abundance and distribution of amphibians in the whole habitat. This gives an idea of how many are in that area, but you might not know how many are in the whole place. Using both methods together gives a better idea of the amphibian population in an area.
Strengths and challenges
These labor-intensive methods are relatively cheap and easy. However, they only give a great return in numbers when there are high densities of amphibians.
Normativity
Drift fences may not provide accurate population estimates for species that are skilled climbers or jumpers. Additionally, very small individuals, such as metamorphs, may conceal themselves close to the fence and be easily missed. The main issues of transect and patch sampling are that individuals of highly active species may flee before they can be sampled, leading to an underestimation of their numbers. Moreover, these methods only yield good numbers when there are high densities of amphibians. However, the plots should still be randomly distributed and not only in places that look good for amphibians. For Trapping, distinct behavior and location of males and females at a breeding site may result in a significant disparity in the number of each sex caught, resulting in an inaccurate estimate of the sex ratio.
Outlook
Amphibian populations are endangered by habitat fragmentation and spread of Batrachochytrium dendrobatidis, a cutaneous pathogenic fungus causing population declines worldwide (Berger et al. 1998, Cushman 2006). For these reasons, amphibians have the highest proportion of vertebrates threatened with extinction (Beebee & Griffiths 2005). Therefore, tracking amphibian populations has strongly gained in importance in nature conservation. In recent years, DNA-based techniques have increasingly been applied for identification of amphibian populations and tracking gene-flow between populations. A method that yields a large amount of DNA is the toe clipping method. However, this method is controversially discussed as it causes a higher number of deaths due to high stress levels and infections of the wounds (Funk et al. 2005). Buccal swabs are a less invasive technique that can still cause pain and stress to the individual. Buccal cells are collected with cotton swabs from the inside of the mouth. Therefore, the mouth needs to be opened with a sterile spatula. This might lead to bleeding (Pidancier et al. 2003). Skin swabbing is the least harmful method. DNA is collected from the skin on the dorsal or ventral side of the individual by using a cotton swab. Prunier et al. (2012) have shown that dorsal swabs perform better than ventral swabs and are a good alternative to buccal swabs as this method improves the trade-off between animal welfare, species conservation and data quality (Prunier et al. 2012). This development towards less harmful sampling methods highlights the trend towards a stronger consideration of ethical aspects in ecological methods for sampling of vertebrate species.In the future, traditional sampling methods will most likely be more and more replaced by less harmful methods as sequencing requires smaller amounts of DNA.
Key publications
Theoretical:
Sutherland, W. J., Editor (1996). Ecological Census Techniques - a Handbook. P. 178-180. Cambridge University Press.
Empirical:
Euliss Jr, N. H., Swanson, G. A., & MacKay, J. (1992). Multiple tube sampler for benthic and pelagic invertebrates in shallow wetlands. The Journal of wildlife management, 186-191.
References
(1) Baird, D. J., & Hajibabaei, M. (2012). Biomonitoring 2.0: a new paradigm in ecosystem assessment made possible by next‐generation DNA sequencing.#
(2) Beebee, T. J., & Griffiths, R. A. (2005). The amphibian decline crisis: a watershed for conservation biology?. Biological conservation, 125(3), 271-285.
(3) Berger, L., Speare, R., Daszak, P., Green, D. E., Cunningham, A. A., Goggin, C. L., ... & Parkes, H. (1998). Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences, 95(15), 9031-9036.
(4) Cushman, S. A. (2006). Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biological conservation, 128(2), 231-240.
(5) Funk, W. C., Donnelly, M. A., & Lips, K. R. (2005). Alternative views of amphibian toe-clipping. Nature, 433(7023), 193-193.
(6) Nichols, S. J., Kefford, B. J., Campbell, C. D., Bylemans, J., Chandler, E., Bray, J. P., ... & Furlan, E. M. (2020). Towards routine DNA metabarcoding of macroinvertebrates using bulk samples for freshwater bioassessment: Effects of debris and storage conditions on the recovery of target taxa. Freshwater Biology, 65(4), 607-620.
(7) Pidancier, N., Miquel, C., & Miaud, C. (2003). Buccal swabs as a non-destructive tissue sampling method for DNA analysis in amphibians. Herpetological Journal, 13(4), 175-178.
(8) Prunier, J., Kaufmann, B., Grolet, O., Picard, D., Pompanon, F., & Joly, P. (2012). Skin swabbing as a new efficient DNA sampling technique in amphibians, and 14 new microsatellite markers in the alpine newt (Ichthyosaura alpestris). Molecular Ecology Resources, 12(3), 524-531.
(9) Stein, E. D., White, B. P., Mazor, R. D., Jackson, J. K., Battle, J. M., Miller, P. E., ... & Sweeney, B. W. (2014). Does DNA barcoding improve performance of traditional stream bioassessment metrics?. Freshwater Science, 33(1), 302-311.
(10) Sutherland, W. J., Editor (1996). Ecological Census Techniques - a Handbook. P. 178-180. Cambridge University Press. 172-176.
The author of this entry is Anna-Lena Rau.